Abstract
Warm type autoimmune hemolytic anemia (wAIHA) is a rare disease characterized by variable severity and bone marrow (BM) compensation, unpredictable relapses and several complications (i.e. infections and thrombosis). Most therapies are aimed at restoring immune tolerance by targeting various immunologic mechanisms (autoantibody production, reticuloendothelial phagocytosis, and complement activation). BM composition during acute and chronic/relapsing disease phases is still under investigated, and preliminary studies showed that it may predict anemia severity and treatment response.
We aimed at dissecting BM environment by single cell RNA sequencing (scRNA-seq) in patients with wAIHA. We selected 2 patients experiencing mild hemolytic reactivations handled with low dose steroids (M-AIHA) and 2 with severe relapses requiring high steroid doses and rituximab (S-AIHA). We focused on lymphoid/myeloid subpopulations and their gene expression and evaluated the association of output data with clinical features.
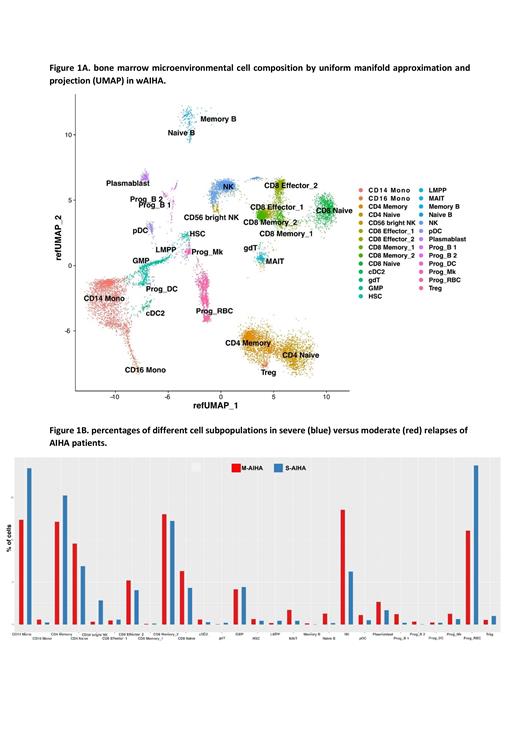
We performed scRNA-seq for 17,989 single cells, detecting over 23,446 expressed genes per cell on average. Uniform manifold approximation and projection (UMAP), a method for nonlinear dimensionality reduction, showed the microenvironmental cell composition, including T-, B- and NK- lymphocytes and their subpopulations, plasma cells, CD14+ and CD16+ monocytes, hematopoietic stem cells, and myeloid precursors (Figure 1A). On the whole, BM microenvironment showed a high frequency of innate immunity effectors such as NK cells and monocytes (11% and 15% of total cells), likely reflecting the inflammatory state typical of autoimmune/autoinflammatory response. T-cell subpopulations were also highly represented. Specifically, more CD4+ memory than CD4+ naïve T-cells (58% vs 38%) were found, and T-regs represented a small fraction (4%). Also, CD8+ memory cells were more frequent than CD8+ naïve and CD8+ effectors (55% vs 24% vs 21%). Both CD8+ memory and effectors type 2 cells were higher than type 1 cells, indicating a likely participation of T cell compartment in disease phenotype. Finally, B cells were particularly underrepresented, probably due to recent therapy (steroids/rituximab). Figure 1B displays % of BM immune cells divided into M-AIHA and S-AIHA. S-AIHA patients showed higher CD14+ monocytes (57% vs 43%) and decreased NK cells (19% vs 81%) as compared to M-AIHA. Interestingly, within the latter compartment, the CD56 bright NKs were over-represented in S-AIHA (83% vs 17%), suggesting an attempt to negatively regulate activated lymphocytes. Moreover, S-AIHA showed a severe decrease of B cells as compared to M-AIHA, consistently with more recent rituximab treatment. Furthermore, we performed differential expression and gene set enrichment (GSEA) analysis within the different cell subset comparing M-AIHA and S-AIHA. Concerning T cells, we found differential expression of genes related to T-cell receptor, immunoglobulins and interferon alpha/gamma response. Regarding CD14+ monocytes, we observed a downregulation of pathways related to immunomodulatory/inflammatory cytokines, complement activation and apoptosis in S-AIHA versus M-AIHA. Finally, using the CopyKat tool, we found aneuploidies in myeloid cells, including stem cells, suggesting that the selective pressure from the immune environment may lead to accumulation of genetic lesions in chronic S-AIHA. This clonal evolution can possibly explain the clinical overlap with myeloid neoplasms.
Overall, these preliminary data show for the first time that scRNA-seq technology is feasible in wAIHA patients and gives insights in the pathogenic role of bone marrow immunologic microenvironment. Additionally, BM composition appears to dynamically modify according to disease severity and treatment, potentially enabling tailored therapies.
Fattizzo: Kira: Speakers Bureau; Alexion: Speakers Bureau; Novartis: Speakers Bureau; Momenta: Honoraria, Speakers Bureau; Annexon: Consultancy; Apellis: Speakers Bureau; Amgen: Honoraria, Speakers Bureau. Bianchi: Agios pharmaceutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Bolli: Celgene/BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Honoraria; Amgen: Honoraria. Barcellini: Alexion Pharmaceuticals: Honoraria; Incyte: Membership on an entity's Board of Directors or advisory committees; Agios: Honoraria, Research Funding; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal